Functional Biomaterials: Tunable Multiblock Hybrid Copolymers as Protein Mimetics
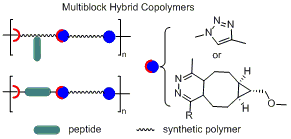
We have created polymer/peptide multiblock hybrid copolymers containing multiple repeats of functional peptides derived from the natural proteins as protein mimetics for biomedical applications. For example, elastin mimetic hybrid polymers (EMHPs) composed of poly(ethylene glycol) (PEG) alternating with an alanine–rich, lysine–containing peptide [(AKA3KA)2, AK2], with or without the cell–binding domains, were synthesized by condensation polymerization employing copper (I) catalyzed alyne–azide cycloaddition (CuAAC) reaction. Covalent crosslinking of [PEG–AK2]n through the lysine amines afforded cell–adhesive, elastomeric hydrogels. Separately, EMHPs consisting of poly(acrylic acid) (PAA) alternating with a elastin–based peptide [(VPGVG)2, VG2] have also been successfully synthesized under CuAAC conditions. [PAA–VG2]n self–assembled into discrete nanoparticles through the concerted interactions between the constituent building blocks. Recently, ultrahigh molecular weight multiblock hybrid copolymers were synthesized employing tetrazine ligation, an ultrafast cycloaddition of s–tetrazine (Tz) with trans–cyclooctene (TCO) derivatives. The modular approaches allow facile substitution of the polymer and peptide segments to fine–tune the materials properties for applications in tissue engineering and drug delivery.
Collaborators:
Selected Publications: