Functional Biomaterials: Mechano–Responsive Hydrogels
Recent biophysical studies using nanotools revealed a diverse set of structural motifs in ECM that could change conformation over a range of mechanical forces. Such force–induced changes ultimately produce changes at the biochemical level that effectively direct cellular behaviors. Synthetic biomaterials with the ability to respond rapidly and reversibly to mechanical stresses over prolonged periods of time in the human body have yet to be developed. To mimic the modular domain structures of functional proteins in natural ECM, we are exploring the use of specific and reversible supramolecular interactions in combination with well–defined polymer synthesis and surface engineering methodologies to construct dynamic and mechano–responsive materials. We anticipate that the unique hierarchical design combined with well–defined polymer chemistry will give rise to materials that exhibit a combination of strength, reversibility, and energy absorption as seen in their natural analogues. The ability to systematically vary the matrix mechanical properties is likely to offer handles for the determination of cell behaviors.
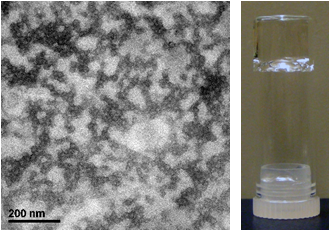
We have recently created a new type of hydrogel material using self–assembled block copolymer micelles (BCMs) as the dynamic building blocks and microscopic crosslinkers. Crosslinkable BCMs (xBCMs) were assembled from amphiphilic block copolymer of hydrophobic, rubbery poly(n–butyl acrylate) (PnBA) and 2–hydroxyethyl acrylate–modified poly(acrylic acid) (PAA). Radical polymerization of acrylamide in the presence of micellar crosslinkers gave rise to elastomeric hydrogels whose mechanical properties can be tuned by varying the xBCM composition. Transmission electron microscopy (TEM) imaging revealed that the covalently integrated BCMs underwent strain–dependent reversible deformation. A model hydrophobic drug, pyrene, loaded into the core of xBCMs prior to the hydrogel formation, was dynamically released in response to externally applied mechanical forces, through force–induced reversible micelle deformation and the penetration of water molecules into the micelle core, leading to the weakening of hydrophobic association between pyrene and the micelle core. Covalent integration of dexamethasone (DEX)–loaded xBCMs in hyaluronic acid (HA)–based networks resulted in hydrogels with anti–inflammatory properties and strain–induced acceleration of DEX release.
Collaborators:
Selected publications