
Fact Sheets And Publications

Delaware Gardener’s Guide to Lawn and Landscape Fertilizers
Revised March 2025 | Written by: Amy L. Shober
Introduction
While the soil generally provides most of the nutrients needed for optimum plant growth, there are times when fertilizers are needed. Fertilizers contain one or more essential plant nutrients and can be applied to landscapes to improve plant growth and quality or to correct a nutrient deficiency. There are many fertilizers available to consumers at local lawn and garden centers.
With so many choices, it is easy to get confused. This publication provides information about fertilizers and associated fertilizer terminology to help consumers make educated decisions when purchasing fertilizers and to use them properly in the home landscape or garden. Proper use of fertilizers can result in cost savings for the consumer and reduce nutrient loads in runoff to sensitive water bodies. Although nutrients are essential for plant growth, “too much of a good thing” can negatively impact the health of our waterways.
The University of Delaware’s Soil Testing Program and other regional soil testing laboratories offer low-cost soil testing services for consumers and commercial landscape professionals to evaluate the fertility status of the soil and provide recommendations for the selection and best management of fertilizers and lime to enhance landscape performance.
What Fertilizers are Available?
There is no easy answer to this question because there are many different fertilizers available to the homeowner or gardener for use on landscape plants, lawns, and vegetables. Fertilizer selection will vary depending on geographic location and retailer. For the most part, garden centers and retailers sell fertilizer blends, which consist of a mixture of several nutrient sources that create a specialized fertilizer product. The specific materials used to create the fertilizer blend are identified by the words "derived from" on the fertilizer label. Figure 1 shows a generic fertilizer label, which is like what you will see on commercially available fertilizers for sale in Delaware. In Delaware, the fertilizer label must include the following information:
The net weight;
The brand and grade;
The guaranteed analysis;
The name and address of the registrant.
Blends are created to achieve a specific fertilizer grade. The fertilizer grade is the percent (by weight) of nutrients in the fertilizer. The three numbers in the grade represent nitrogen (N), phosphorus (P, as P2O5), and potassium (K, as K2O). Please note that only N is listed in the elemental form; P and K are listed on an oxide basis (an artifact of historical weighing methods for determining P and K content in fertilizers). For example, a 10-5-5 fertilizer has 10% N, 5% P2O5, and 5% K2O. In a 50-pound bag of 10-5-5 fertilizer, there are 5 pounds of N, 2.5 pounds of P2O5, and 2.5 pounds K2O. The P content of P2O5 is 44% and the K content of K2O is 83%. Therefore, a 50 lb bag of 10-5-5 fertilizer contains 5 pounds of N, 1.1 pounds of elemental P, and 2.05 pounds of elemental K (Table 1). The guaranteed analysis of N, P2O5 and K2O are required; guarantees for other nutrients (expressed in the form of the element) and the sources of such other nutrients may be included as a parenthetical statement on the label.
Table 1. The amount of nutrients in a fertilizer can be determined based on the fertilizer grade and the weight of the fertilizer bag.
| Nutrient s in a 50 lb bag of 10-5-5 fertilizer | Nitrogen | Phosphorous | Potassium contain secondary plant nutrients | ||
|---|---|---|---|---|---|
| P2O 5 | Elemental P | K2O | Elemental K | ||
| Percenta ge of fertilizer nutrient in bag (%) | 10 | 5 | 2.5 | 5 | 2.5 |
| Weight of fertilizer nutrients in 50 lb bag (lb) | 5 | 2.5 | 1.1 | 2.5 | 2.1 |
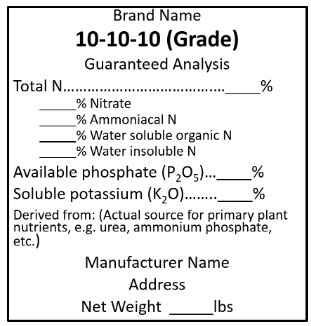
Many of the products sold for use on ornamentals are complete fertilizers. For a fertilizer to be classified as complete, it must contain N, P2O5, and K2O. For example, a 24-8-16 grade fertilizer (as shown in the label below) is a blended fertilizer that is considered a complete fertilizer. Complete fertilizers can also contain secondary plant nutrients (also called secondary macronutrients) and micronutrients. Secondary plant nutrients include calcium (Ca), magnesium (Mg), and sulfur (S). The micronutrients manganese (Mn), boron (B), iron (Fe), zinc (Zn), copper (Cu), molybdenum (Mo), and chloride (Cl), are required by plants in smaller amounts than the primary and secondary plant nutrients. Some fertilizer materials contain one or more of these secondary plant nutrients or micronutrients; these additional nutrients will be listed on the fertilizer label.
While it was common in the past for turf fertilizers to be complete, nutrient management concerns related to P (i.e., too much P causing pollution of water bodies) in our region have led manufacturers to reformulate blends for use on turfgrass. Now you can purchase P free fertilizers for use on established turfgrass. Starter turf fertilizers may be needed to promote healthy root growth in newly established lawns; these products may be available at some outlets, but the guaranteed amount of P2O5 in the formulation will likely not exceed 2%. This shift in fertilizer availability is largely related to regulatory changes in other states that dictate fertilizer label requirements for fertilizers labeled for use on turfgrass. The Delaware Nutrient Management Law (3 Del. C. §2250) prohibits P applications to turfgrass unless P is recommended based on the results of a soil test or P is being used to establish, re-establish, or repair turf areas.
Fertilizers available to consumers for use in the landscape will also have variable properties depending on brand and formulation. Many fertilizer materials are inorganic or synthetic. Examples of inorganic nutrient sources that may be used in blends include urea, ammonium sulfate, ammonium phosphate, and potassium chloride (e.g., muriate of potash). Other fertilizer materials are organic in nature; these include animal manures, such as guano or bone meal, composted materials, and plant residues.
In addition, fertilizer materials may be water-soluble (soluble or quick-release), slow-release, or controlled-release. Water-soluble fertilizer material dissolves immediately in water. Once dissolved, nutrients in the fertilizer are available for uptake by plant roots or leaves (if applied as a foliar application). Most of the inorganic fertilizer materials are soluble unless they have been formulated to be slow or controlled-release. Slow-release fertilizers will release nutrients slowly for a period of time, ranging from weeks to months. Examples of commercial slow-release fertilizers are sulfur-coated urea (SCU), methylene urea (MU), and Nitroform®. Organic fertilizers also contain slow-release nutrients; however, not all nutrients in organic fertilizers are slow-release. Soil microbes must convert these slow-release fertilizer nutrients to forms that can be used by plants. In contrast, controlled-release fertilizer materials are soluble inorganic fertilizers that have been modified to allow nutrients to be released over a specific time period. In a controlled-release fertilizer, the water-soluble fertilizer materials, most commonly urea, are encapsulated in a plastic or polymer coating. Nutrients are released with time; the amount of time it takes for nutrients to be fully released depends on the thickness of the coating, contact with moisture, and temperature. Typically, nutrient release from controlled-release fertilizers increases with increasing temperature and soil moisture. Examples of controlled-release materials are Osmocote®, Nutricote®, and Polyon®. Oftentimes you may encounter fertilizers that are coated with both a polymer and sulfur in order to achieve a desired release rate.
Reading the Fertilizer Label
Now that you know the types of materials available to you, let's discuss how to read the label. You should always read the label thoroughly so you can make the most informed decision possible. This document will go through two examples of materials that can be purchased at a typical "big-box" retailer. Industry distributors who cater to lawn and landscape professionals will have a wider variety of fertilizers to choose from and they can custom blend fertilizers for your particular needs.
Example 1: All-Purpose Fertilizer
The first example is an "all-purpose" ornamental fertilizer listed for use on all flowers, trees, shrubs, vegetables, and houseplants (Figure 2). The label on the box lists that it is a water-soluble fertilizer, so we know the material will dissolve in water and all nutrients will be in a plant-available form. The grade is 24-8-16, which means, by weight, it contains 24% N, 8% P2O5, and 16% K2O. It has a fertilizer ratio of 3 parts N to 1 part P to 2 parts K. (To determine the fertilizer ratio from the fertilizer grade, divide all numbers on the grade by the lowest number. For example, for a 24-8-16 fertilizer, divide all numbers by 8 to get a 3-1-2 grade.)
Figure 2. Fertilizer label for an All-Purpose ornamental fertilizer.
According to the label, 3.5% of the total N in the box is ammoniacal N (ammonium), and the remaining 20.5% is urea (3.5% + 20.5% = 24% total N). In the "derived from" statement, ammonium sulfate, urea, and urea phosphate are the sources of N. (Note: Fertilizers containing ammoniacal N or urea should be watered in to prevent N loss to the atmosphere). The sources of P are potassium phosphate and urea phosphate. The sources of K are potassium phosphate and potassium chloride.
This fertilizer also contains the following plant micronutrients: 0.02% boron (B) as boric acid, 0.07% copper (Cu) as copper sulfate, 0.15% iron (Fe) as iron EDTA, 0.05% manganese (Mn) as manganese EDTA, 0.0005% molybdenum (Mo) as sodium molybdate, and 0.06% zinc (Zn) as zinc sulfate. The term EDTA in iron and manganese EDTA indicates that the nutrient is in chelated form. Chelated forms of nutrients exist as a complex molecule containing carbon and hydrogen that have a prolonged period of plant availability. The other elements are listed as water-soluble.
Example 2: Starter Lawn Fertilizer
Our second example fertilizer is a complete lawn starter fertilizer that contains controlled-release N (Figure 3). The grade is 18-24-12, which means that, by weight, it contains 18% N, 24% P2O5, and 12% K2O. It also has a fertilizer ratio of 1.5 parts N to 2 parts P to 1 part K.
Figure 3. Fertilizer label for a turf starter fertilizer.
According to the label, 9% of the total N is ammoniacal N (ammonium), and the remaining 9% is urea N, both of which are water-soluble forms of N (9% + 9% = 18% total N). In the "derived from" statement, polymer coated sulfur coated urea and ammonium phosphate are the sources of N. However, the normal quick-release action of water-soluble N is tempered by the two fertilizer coatings in this case (polymer and sulfur). Note the statement (below the "derived from" statement) indicating the percentage of nutrients that are coated and thus in controlled- release form. In this fertilizer, half of the total N is controlled release (9%). The source of P2O5 is ammonium phosphate and the source of K2O is muriate of potash (which is potassium chloride). This fertilizer also contains the following plant nutrients: 2.6% sulfur (S) from the sulfur coating on the urea and 9% chlorine (Cl) from the potassium chloride (muriate of potash).
Before Choosing a Fertilizer – Soil Test!
Before you decide to use a fertilizer, you should have your soil tested. The results of a full soil test will help you decide what amount of fertilizer or lime, if any, will benefit your plants. The University of Delaware (UD) Soil Testing Program offers soil testing services to home gardeners for a moderate fee. There are several ways to obtain a soil test kit. All Delaware Cooperative Extension Offices and the UD Soil Testing Program office in Newark sell the kits on-site.
Prepackaged versions of the kit, including a payment form that enables the client to submit payment when returning the sample, are available for pickup at several locations, including garden centers in Delaware, Maryland, and New Jersey. Kits can also be ordered online from the UD Soil Testing Program website and mailed directly to you. All kits come with a soil bag, sample information sheet, instructions for proper sample collection and how to return the kit to the laboratory for analysis. More information about soil testing and how to take a soil sample are available on the UD Soil Testing Program website.
The University of Delaware Soil Testing Program’s basic soil test includes measurement of soil pH, lime requirement, organic matter content, primary plant nutrients (P and K), secondary plant nutrients (Mg, Ca, and S), and micronutrients (B, Mn, Zn, Cu, and Fe). The soluble salts test is also available for an additional fee. The pH and lime requirements tests will tell you if you need to add lime to decrease the acidity of your soil.
The routine soil test does not include a test for soil N. The N cycle in our soils and humid climate is very dynamic; there are no reliable soil tests for predicting N release over the growing season in this region. Instead, N recommendations are made based on the annual N needs of the plant(s) as specified by the client on the soil information sheet, which is submitted to the lab with the sample.
Once testing is complete, you will receive a soil test report (Figure 4); this example report provides recommendations for the maintenance of a bluegrass/fescue lawn. Before selecting a fertilizer, it is important that you read the entire soil test report. The report will include important information about application methods, rates and timing. The report may also reference soil test notes; if so, printed copies of the notes will be included with your report. These notes contain additional important information related to your needs.
It is important that you follow the recommendations on your soil test report and do not apply more fertilizer than specified. The concern here is that if applied at rates that exceed plant needs, N and P can be lost during leaching or runoff events and contribute to water quality problems. Please note that fertilizers labeled for use on turf will have a different ratio of N, P, and K than fertilizers labeled for ornamental plants. Landscape outlets stock P free fertilizers to allow homeowners to be more precise about the amount of P applied to home landscapes. If the P or K levels from your soil test are excessive, buy a fertilizer that only contains N and apply according to the N recommendations in your report. Although excess K is not a water quality concern, it can negatively impact plant health. Potassium fertilizers are mined, and there is a limited supply, so conserve when not in need.
Final Thoughts on Landscape Fertilizers
Begin by having your soil tested to determine the need for fertilizers, and always follow University of Delaware fertilizer recommendations. Knowing fertilizer terminology and understanding the fertilizer label will help you make informed choices if you decide to use fertilizer in your home landscape or vegetable garden. Since improper fertilizer use can contribute to water quality problems, always follow best management practices (BMPs) described in Delaware lawn and landscape publications on the Delaware Livable Lawns website. Guidelines for proper fertilizer application include consideration of plant type, soil type, nutrient quantities found in the soil, timing of application based on season and precipitation, and location of application (avoiding hard surfaces) to minimize waste and runoff potential. You may also contact your local Cooperative Extension Office or see the UD Extension Website for more information about fertilizer recommendations for lawns and landscape plants.
Figure 4. An example soil test report for a home lawn from the University of Delaware Soil Testing Program. The report provides information about soil pH and plant nutrients in the sample and the interpretation of those results. The soil test report also provides recommendations for the application of lime to adjust pH and fertilizers to provide nutrients to landscape plants.
All chemicals should be used in accordance with directions on the manufacturer's label. The use of trade names in this publication is solely for the purpose of providing specific information. University of Delaware does not guarantee or warranty the products named, and references to them in this publication does not signify our approval to the exclusion of other products of suitable composition.
References
Nutrient Management, Delaware Administrative Code Title 3 Section 22. (3 Del. C. §§ 2201-2290). https://delcode.delaware.gov/title3/c022/index.html
Shober, A.L. & Reisinger, A.J. 2021. Soils and fertilizers for Master Gardeners: The Florida gardener’s guide to landscape fertilizers. EDIS Document SL266/MG448. University of Florida – IFAS, Gainesville, FL. http://edis.ifas.ufl.edu/MG448.
About the Author
Amy L. Shober (corresponding author), Professor and Extension Specialist, Nutrient Management, University of Delaware, Newark, DE (ashober@udel.edu)
About this publication
Original publication date: December 2015
Revision date(s): March 2025
Based on original documents prepared by: A.L. Shober for UF-IFAS
Reviewed by
John Emerson, Extension Agent, Turfgrass Management, University of Delaware Cooperative Extension, Newark, DE
Karen Gartley, Director, University of Delaware Soil Testing Program, Newark, DE
UD Cooperative Extension
This institution is an equal opportunity provider.
In accordance with Federal law and U.S. Department of Agriculture policy, Cooperative Extension is prohibited from discriminating on the basis of race, color, national origin, sex, age, or disability.
